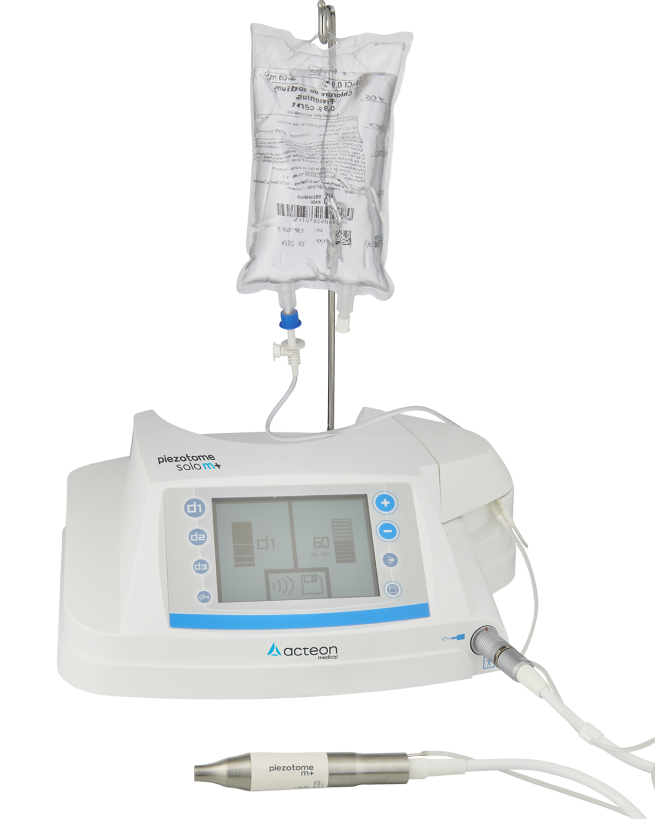
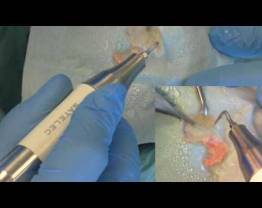
Piezotome® Solo M+
Concentrated ultrasonics for bone surgery in an easy and powerful device
Piezotome® Solo M+, compact and efficient, brings together all of the powerful, reliable and safe components of the M+ range for maximum performance and safety.